Chemical Biology
Chemical Tools for Monitoring and Targeting Fibrosis
Collagens are the most abundant proteins in mammals and the most prevalent constituent of the extracellular matrix. The fibrous proteins provide for stability in disparate tissues and organs and play crucial roles in the modulation of cellular activities. Collagen formation is thus vital for the integrity of skin, tendons, and the tissue in essentially any organ. Excessive collagen formation is, however, characteristic of fibrotic and malignant diseases such as idiopathic pulmonary fibrosis, MASH, diabetes-associated renal fibrosis, and cancer.
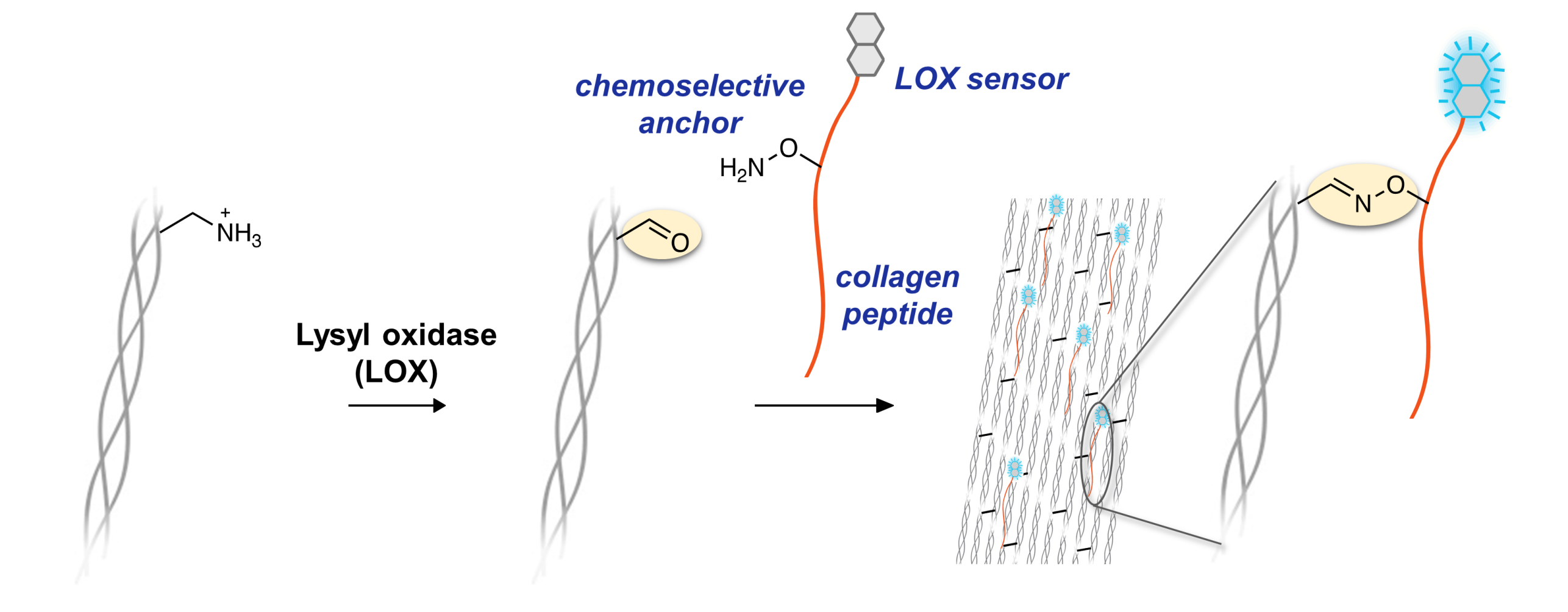
We have established a reactive collagen peptide-sensor conjugate for targeting and monitoring collagen production in vivo and in histological samples. The system consists of a) a turn-on fluorescent sensor for lysyl oxidase, a critical enzyme during collagen synthesis in nature, b) a collagen mimetic peptide, and c) an aminooxy group for chemoselective reaction with the aldehydes created by lysyl oxidase. In vivo and ex vivo studies with histological sections revealed that our collagen peptide-sensor probe lights up newly formed collagen with an unprecedented level of specificity and spatial resolution in tumors, wounds, and other fibrotic tissues.
We are currently a) expanding the tool kit of chemoselective lysyl oxidase sensors, b) interrogating the selectivity of LOX and LOX-induced collagen cross-linking, and c) developing functionalized probes towards disease diagnosis and treatment.
This research is part of the external page Skintegrity Network.
Bioorthogonal Ligations with Isonitriles
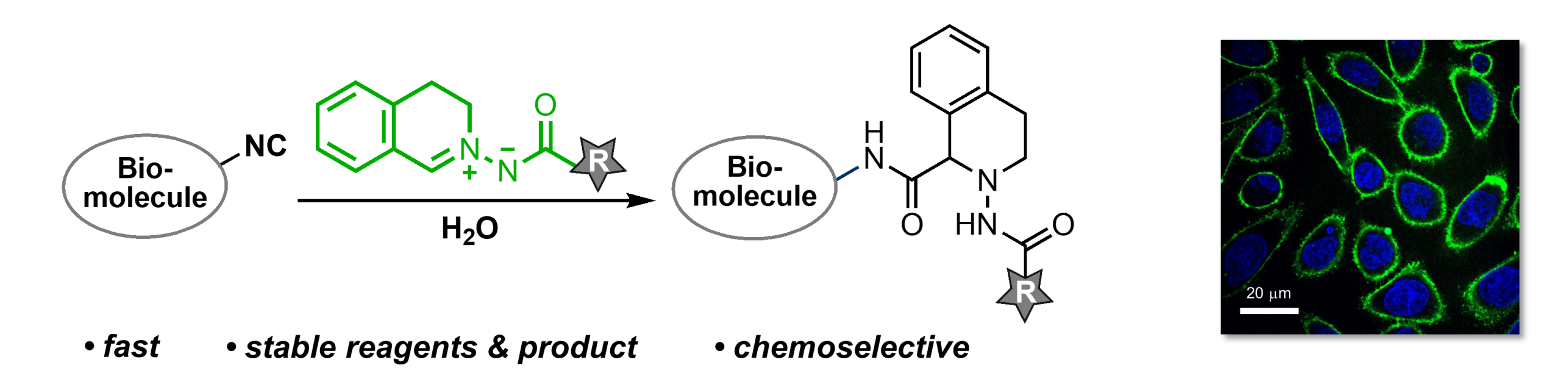
We are developing powerful methods for chemoselective reactions with isonitriles. The isonitrile group is small and, thus, an attractive chemical reporter for biorthogonal ligations. In addition, many natural products contain isonitrile groups but remain undiscovered due to the difficulty of selective labeling. Originally, we used chlorooximes for the chemoselective reaction with isonitriles, research that allowed for the isolation and characterization of minute quantities of isonitrile sesquiterpenoids from a marine sponge. More recently, we introduced azomethine imines (AMI) into chemical biology. These readily accessible 1,3-dipoles are highly effective reagents for the biorthogonal, pH dependent ligation with isonitriles. The ‘AMI-isonitrile ligation’ is orthogonal to established ligations (e.g., SPAAC and iEDDA reactions) and compatible with live cells.
We are now optimizing the ‘AMI-isonitrile ligation’ further and are applying this chemoselective reaction as a tool for chemical biology, material sciences, and medicinal chemistry.
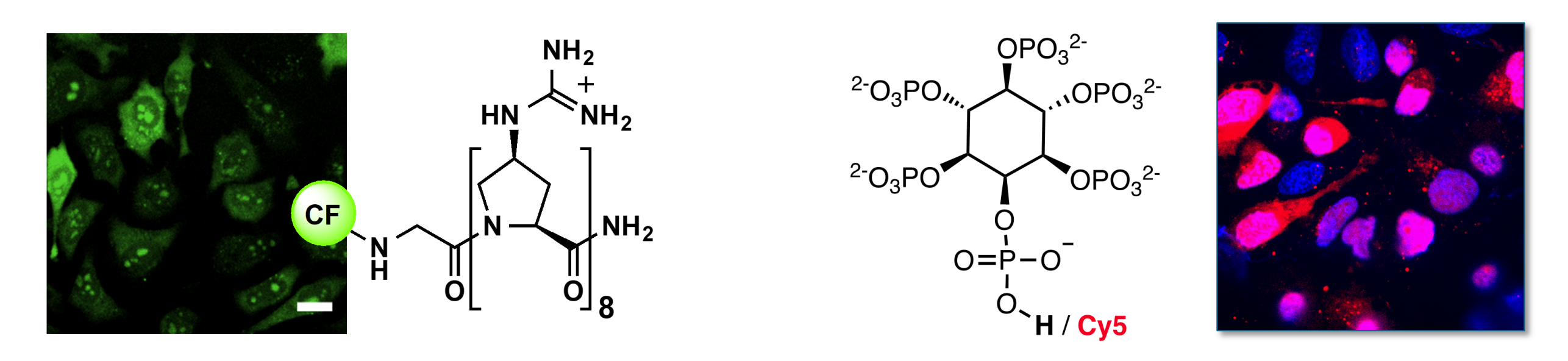
Cell-penetrating Peptides for Intracellular Targeting
Cell‐penetrating peptides (CPPs) are a promising tool for non‐invasive delivery of therapeutic or diagnostic molecules into mammalian cells. We have shown that preorganization of cationic charges in distances of 9 Å along an oligoproline backbone enhance the cellular uptake of CPPs into various cancer cells compared to more flexible oligoarginines with undefined charge display. We also showed that the higher cellular entry of the CPPs with preorganized positive charges correlates with their higher binding affinity to anionic cell‐surface glycans. In addition, our cationic oligoprolines localize in the nucleus and feature high proteolytic stability as well as low cytotoxicity.
We are currently using our cationic oligoprolines for the delivery of cargo (e.g., siRNA and inositol phosphates) and the development of organelle-specific CPPs.