Supramolecular Chemistry
Collagen-based Biocompatible Materials
Collagen is the most abundant protein in mammals and the most prevalent constituent of the extracellular matrix. It provides for stability in disparate tissues and organs and plays crucial roles in the modulation of cellular activities.
Through the synthesis and study of collagen model peptides our research has yielded a deep understanding of the factors that govern the conformational stability of collagen at the molecular level. We have utilized this knowledge to establish pH-responsive, hyperstable, and heterotrimeric synthetic collagen. We also established a reactive collagen peptide-sensor conjugate for targeting and monitoring collagen production in vivo and in histological samples.
Currently, we are designing synthetic collagen peptides to tackle challenges in tissue engineering and wound healing. In particular, we create collagen-based materials that can 1) influence the growth and protein expression of fibroblasts, 2) modify the mechanical properties of collagenous tissue, and 3) integrate into wounds, tumors and other tissue abnormalities to promote repair.
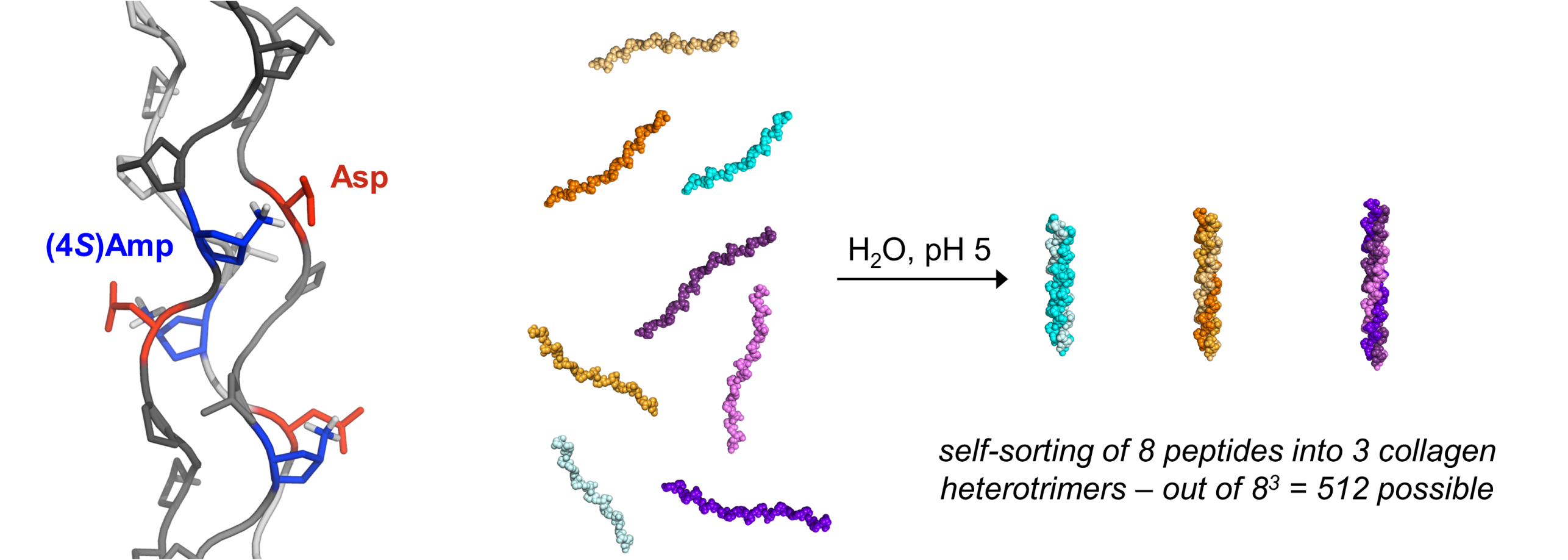
(Non)-Covalent Peptide Frameworks
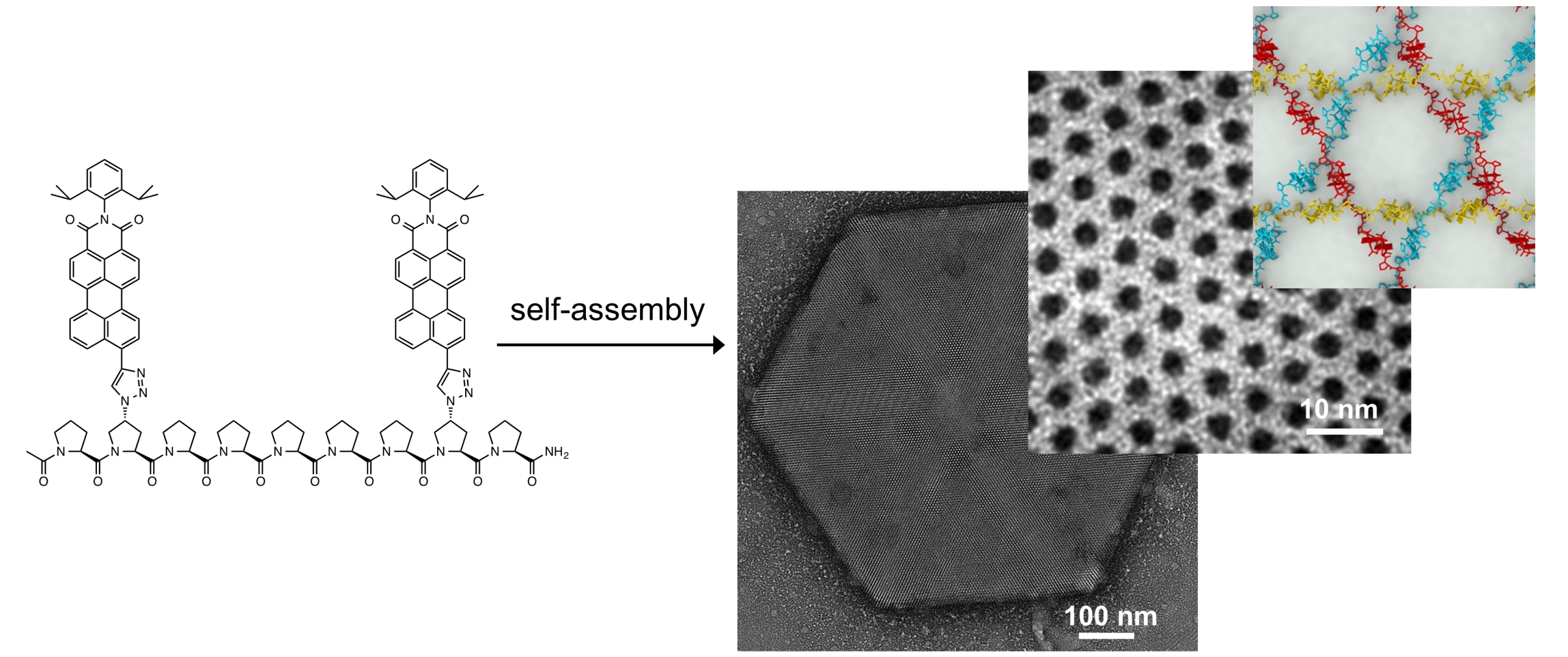
Our group uses oligoprolines as rigid and easy-to-functionalize molecular scaffolds to create and control the morphology of nanostructured materials. We developed, for example, conjugates between oligoproline and π-conjugated systems that form hierarchical self-assemblies with morphologies that can be tuned by seemingly subtle modifications at the molecular level. This approach established the first extended triaxial supramolecular weave. This non-covalent micrometre-scale framework is held together solely through the interplay of weak non-covalent interactions. Similarly to woven fabrics, the material is more robust than non-woven self-assemblies that consist of the same building block. The uniform hexagonal pores of the interwoven network are versatile cavities for hosting guests, e.g. iridium nanoparticles.
Building on the detailed understanding of the supramolecular weave, we are currently expanding this framework towards guest sensing and catalysis and are designing novel molecular architectures.
Peptides for Metal Nanoparticle Formation
We use peptides to generate noble metal nanoparticles with control over their size and shape. Colorimetric combinatorial assays allowed us to identify tripeptides that control the formation of mono-disperse, water-soluble Ag-, Pd-, Pt-, and AuNPs in defined sizes. Structure-activity relationship studies revealed a specific role of each amino acid for the formation and stabilization of the nanoparticles. Our research yielded, for example, peptide‐stabilized platinum nanoparticles with greater toxicity against hepatic cancer cells than against other cancer cells and non‐cancerous liver cells.
We are currently expanding the methodology to the formation of other types of nanometer-sized metal composites and applications in catalysis and biomedicine.
